Carbon is an element found in all forms of life. Carbon makes up 18 percent of our bodies and is a
major component of trees and plants. It also exists in the environment in non-living things like rocks, oil,
natural gas, coal, and air. In short, it is the basic building block of all life and the environment we
live in. Carbon, in its many forms, is exchanged among the atmosphere, oceans, and land. This is called the
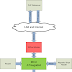
carbon cycle (see Fig. 1). In simple terms, plants take carbon dioxide (CO2) from the atmosphere and
turn it into biomass (wood, leaves, fruits etc.) process called “photosynthesis.” Some of the
carbon taken in by plants is returned to the atmosphere through respiration by the plant or by
other living organisms, including humans, that use it for food or fuel. This renews the carbon cycle. By
extracting fossil fuels (oil, gas and coal) from deep in the Earth, we are overloading the atmosphere with carbon, and changing our climate in irreversible ways.
One critical part of the carbon cycle takes place in forests. Forests exchange large amounts of CO2 and other gases with the atmosphere and store carbon, in various forms, in trees and soils. Carbon stored in plants or soils is called “sequestered carbon.” Carbon returned to the atmosphere when it has been used by trees or other organisms as energy for life is called “respired carbon.” If we follow the fate of carbon in a forest, many processes are occurring .Much of the CO2 in the air above a forest is taken in by trees through the process of photosynthesis, where it becomes one of the building blocks for tree growth or energy for life.Some carbon goes right back into the atmosphere as the tree respires (breathes out); but, if it stays, then it may remain sequestered in the tree throughout its life—whether that is 10 or 500 years.
The Earth’s climate is changing. In the past, the climate warmed and cooled due to natural processes. Now
humans are changing the climate by burning fossil fuels and permanently deforesting landscapes. Many of our
wildlands are being stressed beyond their natural ability to adapt to these dramatic changes, and the full extent
of how to deal with these changes remains unclear.
What is clear, however, is that a better understanding of the carbon cycle and the role forests play will
help us better manage forests and fire in a future marked by climate change. When a tree dies or loses a leaf or branch containing carbon, it generally falls to the forest floor where it will be decomposed by bacteria and fungi, and either be respired back into the atmosphere or made into soil carbon. Carbon is returned daily to
the atmosphere when it is decomposed and respired by soil organisms. But, much of it remains in complex chemical forms that resist decomposition and persist for hundreds to thousands of years. Soil carbon is an important carbon storehouse. It accounts for as much carbon as is presently found in plants and the atmosphere combined.Fire plays an important role in the forest carbon cycle. When a fire occurs, a portion of the trees, plants, grasses and other biomass are consumed and converted to CO2 and other gases, and another portion is converted to charcoal, an essentially permanent form of storage. Only 10 to 30 percent of the biomass in a forest is actually consumed by a fire; the majority remains on-site. Live trees will continue their role in the carbon cycle. Dead trees will slowly decompose and release carbon to the atmosphere or make new soil carbon. Regrowth after a fire will recapture carbon from the atmosphere, reversing the fire’s emissions. About one to 10 percent of biomass killed in a fire is converted to charcoal, a uniquely stable form of carbon that will persist for thousands of years.
continually recycled. Earth’s plants and animals evolved over thousands of years under this level of CO2 and a slowly changing climate, creating the forest ecosystems we know today. Now we are extracting billions of tons of fossil fuels each year to meet the energy demands of a growing global population, adding new carbon to the atmosphere and changing our climate. Prior to fossil fuel use, this carbon was locked underground for millions of years and was not part of the carbon cycle. Current levels of CO2 are 25 percent higher than before the Industrial Revolution. As a result of these elevated levels of carbon, our forest ecosystems are changing. They are changing the way they grow in response to elevated CO2, and they are changing in response to new climate patterns, including warmer temperatures and different levels of precipitation. These changes also affect the way that they store and release carbon, sometimes reducing the amount that goes into tree carbon or soil carbon.


















.jpg)